
The Boston Globe Magazine recently published a story with my byline on sparkling wines for the holidays. I had fun putting it together and editor Anne Nelson made a lovely two page spread of it, but it left me a tiny bit unfulfilled. The assignment — recommend 10 bubblies at a variety of price points worth making merry with and provide a tasting note on each — reminded me that I have long yearned to know more about how it is that those swarms of pearly spheres make their appearance in wine and how exactly they behave.
I’m not talking here about how carbon dioxide (the gas that makes wine fizzy) gets into wine. There are a number of techniques for accomplishing this. Descriptions of the various approaches are easily found online and are easily grasped. For me the mystery has always been how all that CO2 gets cut loose, streams in vertical columns straight to the surface and forming the sudsy cap that subjects the nose and tongue to the lascivious tickling we crave.
Most of what we know about the science of bubbles is resident, as you might imagine, at the University of Rheims located in the heart of Champagne country. But it wasn’t until a young physicist named Gérard Liger-Belair took an interest in examining at very close range what all those bubbles (more than two million in a single glassful) we really up to. His 2004 book, Uncorked: the Science of Champagne, is the first attempt to take a scientific approach to the mysteries of bubbly behavior.
A whole book and a series of scholarly papers on the subject may be more than you have a thirst for, so here are a few highlights – enough to give you plenty to chat about at a holiday party as you stand around with a flute in your hand.
How much carbon dioxide is in a typical bottle of Champagne, anyway? About 5 liters – or about 6 times the volume of the wine contained in a 750ml bottle.
What happens when you pop the cork? Pulling the cork reduces the pressure on the wine and the CO2 dissolved in it. Up to 80% of the CO2 is liberated immediately. It’s the 20% that’s left behind that causes all the commotion.
How are bubbles born? You may have read that it has something to do with minute irregularities in the surface of the glass, but Liger-Belair has shown that they can originate even in very smooth glass within extremely tiny cellulose fibers that fall from the air or are left behind by a towel.
The fibers – which are really shaped like tubes – trap tiny pockets of air as the glass is filled. Carbon dioxide from the wine diffuses into these air pockets making bubbles as if on an assembly line (see high-speed photograph and diagram below). The microfibers themselves consist of chains of polymerized glucose.
Each fiber represents a “nucleation site” — a spot from which a stream of bubbles can be generated. Nucleation sites can be etched in glass to produce more orderly and column-like bubble activity than result from random fiber placement.
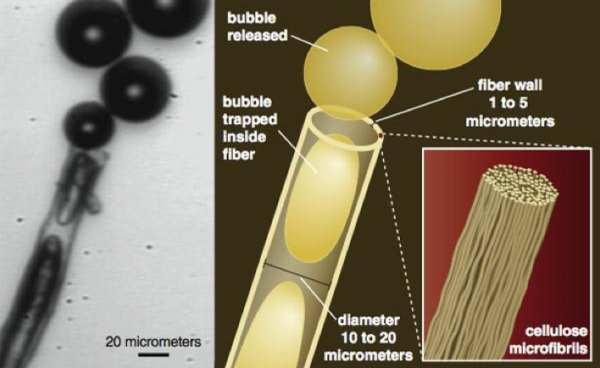
How many bubbles are released in an average glass of sparkling wine? Around two million. They form at a rate of about 30 per second from each nucleation site.
How long does a bubble last? From 1 to 5 seconds, during which time they grow in size to reach a maximum diameter of about 1 millimeter.
Are smaller bubbles more desirable? Some consumers think so, which is why Champagne makers have been revising downward the sugar content of the dosage that’s responsible for the second, in-bottle fermentation. Less sugar for yeasts to ferment means less CO2 is produced. The trend today, Leger-Belair explains, is to use as little as 0.6 oz. of sugar – the lowest permitted by the appellation rules.
Are bubbles just about visual appeal? Leger-Belair’s research has shown that the components in the droplets produced by bursting bubbles on the surface of wine exceed those found lower down by a factor of 30. All that sound and fury carry more of what you love about wine to your nose and palate.
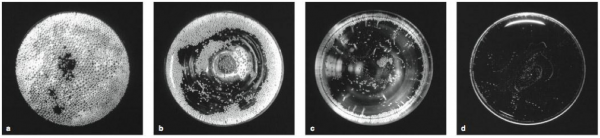
Credit: Guillaume Polidori, Philippe Jeandet, Gérard Liger-Belair. Bubbles and Flow Patterns in Champagne. American Scientist, 2009
Which glass is better for sipping fizz – the broad, shallow coupe or the tall, slender flute? This long-standing controversy may be never really be resolved since each creates a different environment for effervescence to play out. Coupes produce a more explosive CO2 release that offers a dramatic burst of orthonasal olfaction (the kind we experience by sniffing) but dissipates quickly. Flutes generate a more persistent and orderly bead but with less gusto, leaving more CO2 in the wine to bring flavors and aromas into the mouth where the more efficient retronasal olfaction can luxuriate in them.
How should sparkling wine be poured? Start by tilting the glass toward the bottle and let the wine flow quietly along the wall of the glass. Gradually restore the glass to an upright position and crown it with a small, creaming head.
In other words, imagine you’re pretend you’re pouring a Budweiser.
Reach me at stephenmeuse@icloud.com
Follow Stephen Meuse